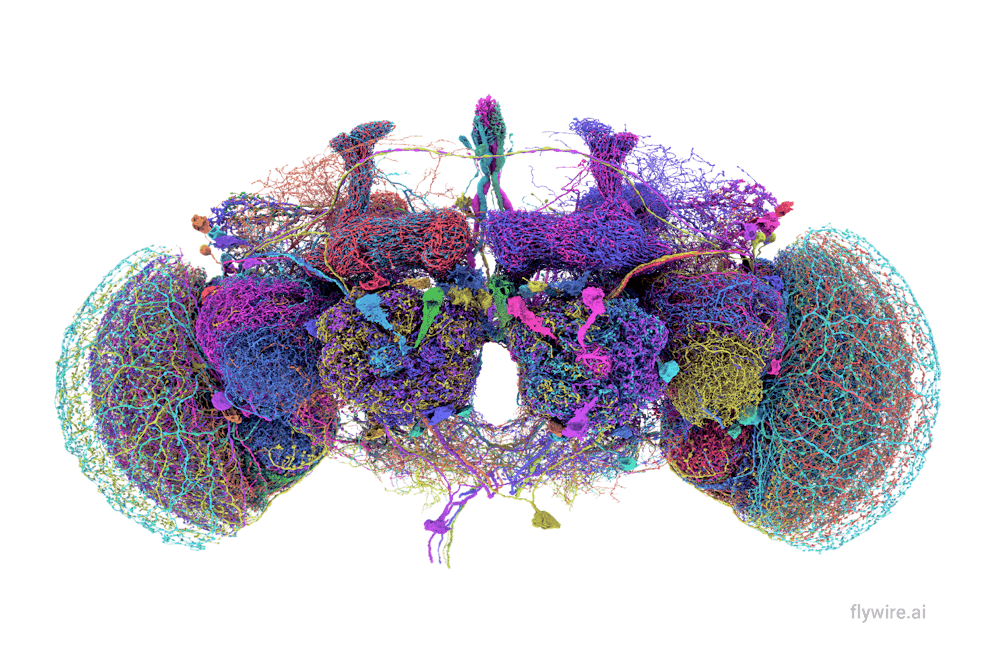
With 140,000 neurons, 50 million synapses and over 100,000 cell label annotations, the fruit fly brain has been mapped and published in a series of papers written by scientists worldwide, including contributors at Brown. It’s a marked advancement in neuroscience that researchers say will improve our understanding of the human brain.
This complete connectome — a diagram mapping every connection between neurons in a brain — for Drosophila, otherwise known as fruit flies, was put together by the FlyWire consortium comprising hundreds of collaborators.
The development is an important step in scaling up brain mapping to larger organisms such as mice and even humans, according to Sven Dorkenwald, a research fellow at the Allen Institute of Brain Science and the University of Washington, who authored the flagship paper with these findings last month.
But brain mapping with this level of detail is difficult. Creating a completely annotated Drosophila connectome that shows each individual synapse — junctions between neurons — took over more than four years.
“Flies have about 130,000 neurons in their brain,” said Karla Kaun, an associate professor of neuroscience at Brown who contributed to the project. “Humans have more connections than there are stars in the Milky Way. And so if you want to study things one neuron at a time, it’s impossible to do that even in a mouse brain at this point.”
Despite being degrees of magnitude less complex than the human brain, the Drosophila connectome serves an invaluable role in understanding “how neurons are connected and how those connections drive neural activity and behavior,” Kaun said. She added that her research leverages the FlyWire connectome as a “roadmap.”
Many of the brain components in flies “are highly conserved,” she added. “So how it works in a fly brain is very, very similar to how it works in our brains.”
Despite its similarity to human brains, the fruit fly brain is far simpler and easier to manipulate, Kaun explained. So, studying it can yield a “fundamental” understanding of cell functionality that can be extrapolated to the human brain.
The Drosophila connectome is like “Google Maps” for the fly brain, said Philipp Schlegel, a senior research associate at the University of Cambridge and the Medical Research Council Laboratory for Molecular Biology, who authored a companion paper to the connectome’s release.
The grayscale, un-annotated images of the connectome are comparable to satellite images of the Earth, he explained. But more information is needed to label the neurons and discern their functionality. Further annotations provide additional details to understand the brain, similar to the building descriptions or restaurant reviews on Google Maps, Schlegel added.
According to Kaun, the interactive connectome would allow users to “pull out which things are attached to which things” for a more complete understanding of how different parts of a Drosophila brain work together.
Different labs use the connectome to understand the bridge between the physical connections of neurons in circuits to the functional impact, Kaun said. But each expert “has their own little thing” that they focus on, including smell, hearing and navigation. In fact, having numerous experts who focus on just a couple of “favorite neurons” ended up being central to the production of FlyWire’s connectome, Dorkenwald and Kaun said.
The first step to completing the connectome was to attain the high resolution image data via electron microscopy. After acquiring images for 8,000 slices of the brain — each of which is 40 nanometers in depth, 0.0005 times the width of a human hair — researchers converted this to a 3D model using artificial intelligence, showing all the neurons and synapses, Schlegel explained. But this needed proofreading.
“AI has gotten extremely good,” but not perfect, Dorkenwald said. “Just a few errors that you make by reconstructing those neurons means that your data is unusable for analysis.”
“The good news,” he added, “is that just a few corrections make it usable.”
In an effort to catch errors and recruit scientists to collaborate on the project, the FlyWire consortium made the connectome open access, so labs could join and correct neurons of interest. Kaun’s lab, for instance, contributed “in a minor way” thanks to the work of one of her former graduate students, Kavin Nuñez ’15 PhD’21, who characterized a few octopaminergic neurons, she said.
This work allowed Kaun’s lab to understand how rewarding alcohol was to hungry flies. “We discovered, using the connectome resources, that there was a hidden neuron,” she said. “It turned out that (it) was the neuron that was responsible for the behavior.”
Even though the FlyWire connectome was only published this year, over 30 publications used the resource before it was even fully annotated, Dorkenwald said.
The fact that “there are so many labs around the world that came in to work together is very unique, and is really at the heart of this project,” he said.
Kaun said the FlyWire connectome exemplifies the value of open science.
“I think it’s setting a new standard on how science should be done,” she added, “and I think that’s really exciting.”

Elise Haulund is a science & research editor and sophomore from Redondo Beach, CA. Concentrating in English and biology, she has a passion for exploring the intersection between STEM and the humanities. Outside of writing, researching and editing, she enjoys ballet-dancing, cafe-hopping and bullet-journaling.