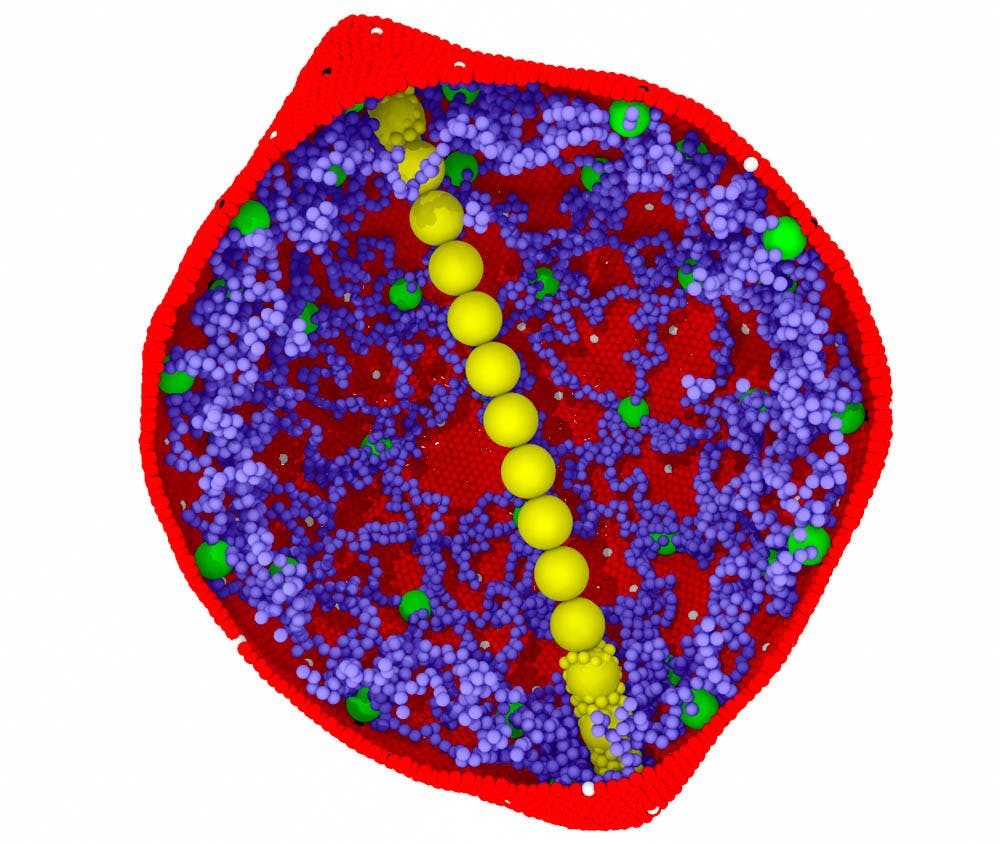
A University research team has developed an innovative computer model that could help researchers design drugs to treat sickle cell disease, which is currently incurable and lacks abundant or effective treatment options.
The team, which primarily focuses on fluid mechanics but was able to apply the same principles to blood, modeled the sickling process of red blood cells.
Sickle cell disease causes a mutation in the oxygen-carrying proteins located in red blood cells, called hemoglobin. The sickling process leads to red blood cells developing a stiff, crescent moon shape, which makes it harder for red blood cells to go through blood vessels throughout the body and causes recurrent pain in individuals with the disorder.
The team of researchers included George Karniadakis, professor of applied mathematics and senior author of the research, Peter Vekilov, physical chemist and professor of chemical and biomolecular engineering at the University of Houston, Lu Lu GS, a PhD student in Karniadakis’ lab and co-author of the research, and He Li, an assistant research professor of applied mathematics. The team also collaborated with researchers at the Massachusetts Institute of Technology and the Massachusetts General Hospital.
The model built on a proposed theory of the way red blood cells are affected by the mutation that accompanies sickle cell disease. This process of sickling follows a two-step nucleation mechanism, which was proposed in earlier research by Vekilov as an expansion to the original 1876 classical theory of nucleation. The two-step nucleation mechanism explains that the nuclei of hemoglobin in red blood cells come together in a disorganized state and in high concentrations. Previous chemical and physical studies have shown that if you have a few hemoglobin molecules, they will form elongated fibers in a process known as nucleation. These fibers push the red blood cell membrane out of its traditional circular shape and eventually lead to the sickle cell shape.
There are currently only two Food and Drug Administration-approved drugs that are aimed at managing pain for sickle-cell disease, though both are not effective at reducing pain episodes for many individuals with the disease.
“The most important thing is to develop a model that looks at the physical and chemical characteristics in red blood cells,” Vekilov said. This makes the group’s model more reliable than one produced in 2017 by another group that did not take into account these differences. This model explicitly accounted for differences that exist within different parts of the body, such as hemoglobin concentrations.
Massachusetts General Hospital provided the team with blood from a number of patients. Hemoglobin was then isolated from the patients’ blood, and MIT researchers observed the hemoglobin elongation process in a controlled oxygen environment.
The team’s model attempted to understand the factors and conditions that influence this sickling process. The group tested their model in four conditions, altering factors like temperature, oxygen, pressure and hemoglobin concentrations. They also tested their model in four drugs that targeted the amount of energy needed to make one cell nucleus and thus result in nucleation. After observing differences based on these conditions, the model analyzed which of the factors was most significant.
“The model helps researchers decide if we design new drugs, which factors we should target,” Lu said.
The group’s computer model was successfully able to replicate a majority of previously collected clinical data, which could allow researchers to avoid costly, time-consuming and often unsuccessful clinical drug trials.
Because the National Institutes of Health funded this research, it has taken charge of sharing the group’s research with the scientific community. Recently, the group has been approached by a company called HemaNext, which provides healthy red blood cells to people for transfusions. HemaNext hopes to incorporate the model in their current testing to ensure that transfusions are safe in all types of conditions.
The group plans to expand the sickle cell project and is currently putting together an application to the NIH. They hope to find ways to better simulate clinical data and find ways to make the model more individualized, since different patients exhibit unique health factors that may influence their sickle cell disease.