Fifty-eight percent of students admitted to the University’s class of 2017 indicated on their applications that they intend to concentrate in the physical or life sciences, with engineering, biology and computer science topping the list of intended concentrations, The Herald reported Monday. Hundreds of those admits will likely enroll in several of the University’s introductory science courses — which Dean of the College Katherine Bergeron described as “an area of concern for many people, both here at Brown and nationally.”
Student interest in science has been gradually increasing: 55 percent of students admitted to the classes of 2016 and 2015 indicated they intended to study physical and life sciences, up from 53 percent in the admitted class of 2014. These increases coincide with the University’s ongoing efforts to boost its science profile, which have included establishing the School of Engineering in 2010, moving the Alpert Medical School to a new building in 2011 and launching a $50 million initiative in 2012 to grow the Brown Institute for Brain Science.
University administrators, professors and students identified introductory science courses as a key area to examine and potentially alter.
This four-part series will explore the diversity of students who choose to enroll in introductory courses for science, technology, engineering and math subjects, why students decide to stop pursuing certain fields of science and potential changes that may be made to the University’s approach to science education.
A national challenge
The University’s emphasis on science education over the past several years has aligned with the goals of the Association of American Universities, which announced in 2011 that it would spend the next five years implementing changes to STEM education in its member universities, a group that includes Brown.
Bergeron said simple concepts in STEM subjects are often the most difficult to teach. “There is really an art to addressing those questions with an audience that may not yet have the full vocabulary,” she added.
Introductory science courses must meet the needs of a large range of students, which makes teaching them challenging, many professors told The Herald.
“People come in with many different goals, many different backgrounds and different interests. You can’t make everyone equally excited all the time,” said Professor of Chemistry Richard Stratt about teaching CHEM 0330: “Equilibrium, Rate and Structure.”
Attracting students
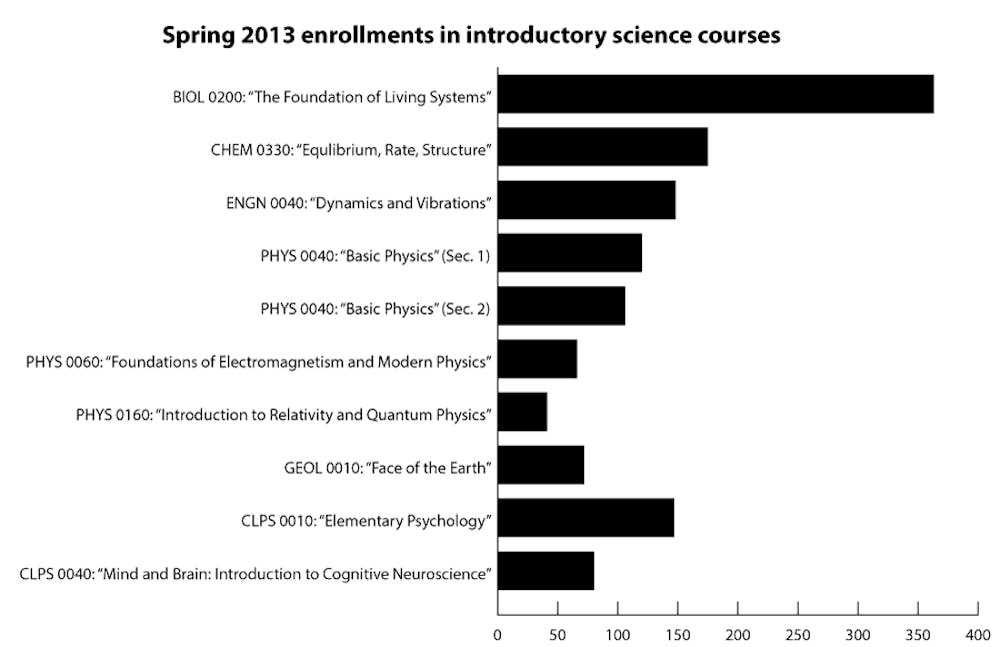
Despite the negativity that may surround large class sizes, students often take large courses as electives, enrollment data indicates, though some lecture courses are more successful than others at catering to a wide range of students.
Each semester, several courses across disciplines enroll hundreds of students. In fall 2011, one section of CHEM 0330 enrolled 278 students, almost all of whom were first-years and sophomores, according to the Critical Review.
Other introductory courses had similarly high enrollment numbers of first- and second-year students, but the data shows the reason students take these courses is not consistent across disciplines.
In spring 2011, nearly 25 percent of students in BIOL 0200: “The Foundation of Living Systems” who responded to the Critical Review survey reported taking the course without using it to satisfy a requirement. That fall, in ECON 0110: “Principles of Economics,” students who reported taking the course without satisfying a requirement made up almost half the class, and in CLPS 0020: “Approaches to the Mind: Introduction to Cognitive Science,” they made up the majority.
But in the one section of CHEM 0330 for which there is Critical Review data from fall 2011, only 6 percent of survey respondents reported that they were not taking the course to satisfy a requirement. In two sections of PHYS 0040 in spring 2011, only 7 percent of respondents said they were taking the course as an elective.
Do lectures work?
The size and lecture format of introductory science courses renders them less effective, said Mitchell Chang, a professor at the University of California at Los Angeles who studies higher education trends. It is harder for professors to cultivate student interest in “impersonal” lectures, he said.
“I think PowerPoint killed teaching,” said Jake Moffett ’15, who has taken introductory courses in biology, chemistry and physics. “Brown advertises, in their stupid pamphlet thing, small class sizes, but they don’t tell you to get to the small class sizes you have to take like 30 500-person classes,” he said.
In large classes like CHEM 0330, “It’s easy to fall through the cracks,” said Victoria Brown ’15, who enrolled in the class last semester.
Bergeron said large class sizes are an inevitable result of having many students interested in particular courses and a limited number of faculty members.
“Given the resources we have, it is what ends up being the most efficient way to educate people,” said Robert Pelcovits, professor of physics. In large lectures, “It becomes a challenge — not impossible, but a challenge — to engage people,” he added.
Senior Lecturer in Neuroscience John Stein PhD ’95 P’13 said he often sees large, introductory courses portrayed in a negative light. But dividing a large lecture course into smaller seminars would require “a ridiculous reallocation of resources.”
Despite the emphasis placed on the importance of small classes, Professor of Comparative Literature Arnold Weinstein said he finds lecturing to be a “remarkable way of organizing material,” though he said he would not advocate lecture courses that were not accompanied by discussion sections.
Dividing and conquering?
Wesley Bernskoetter, an assistant professor of chemistry who taught CHEM 0330 this past fall, said one of the challenges of teaching a large course involves teaching students with a wide range of backgrounds and interests.
He said he tried to balance a rigorous foundation in chemistry for students who planned to advance in the field with some of the subject’s real-world implications to “spark interest in people” who may not consider themselves future chemists.
Some departments have tried to alleviate these challenges by tailoring specific introductory sequences toward different groups of students.
In the Department of Geological Sciences, there are two options for introductory courses, said Jan Tullis, professor of geological sciences. GEOL 0220: “Physical Processes in Geology” is a rigorous course intended primarily for science students, and GEOL 0010: “Face of the Earth” provides students with an introduction to geological concepts without the same level of in-depth explanations.
“Our goal is to attract and engage as high a proportion of undergraduates as possible,” Tullis said.
Physics also has separate introductory tracks for students with different levels of preparation. PHYS 0030 and PHYS 0040: “Basic Physics” are primarily intended for pre-medical school students. PHYS 0050: “Foundations of Mechanics” and PHYS 0060: “Foundations of Electromagnetism and Modern Physics” are designed for students who are interested in physics but do not have much background in the subject. A third sequence is for students who “clearly need a more challenging, higher-level course,” Pelcovits said.
This model is advantageous because potential concentrators are not forced into a large class filled with apathetic students enrolled in order to fulfill a requirement, Pelcovits said.
But chemistry and biology follow a different model, with only one primary introductory course offered for each subject.
Bernskoetter said he thinks “it’s against the Brown model” to divide courses based on student interest in the subjects. Dividing the introductory class would require first-year students to have “self-defined” their areas of interest, which contradicts the spirit of the Open Curriculum, he said.
Chemistry used to have different tracks, said Matthew Zimmt, professor of chemistry and chair of the department. But he added that taking chemistry concentrators and “segregating them from people who don’t know whether or not they are intellectually interested is not necessarily in the best interest of the class.”
Maximizing impact
In addition to the main introductory courses offered in each discipline, the University created small first-year seminars in part to introduce students to certain fields through alternative paths, Bergeron said.
“The challenge has to do with scaling them up,” she said. “It may well be that some of them could be translated quite profitably to a new modality so that the kind of cognitive work that students do when they actually solve problems could be enhanced.”
Bergeron said she thinks the University should implement more courses that involve “‘high-impact practices,” which include writing, research, problem-solving and group work. Unlike listening to lectures and taking exams, these forms of learning “promote active engagement,” she said.
Still, “it is not obvious that by definition, large classes are a problem,” said David Targan, associate dean of the College for science education. “If a faculty member is engaged and students are engaged, size itself is not critical.”
The next story in this series will explore why students enroll in introductory science courses and why courses offered by some departments appeal to a broader range of students.
- Additional reporting by Phoebe Draper and Sahil Luthra
ADVERTISEMENT
More




