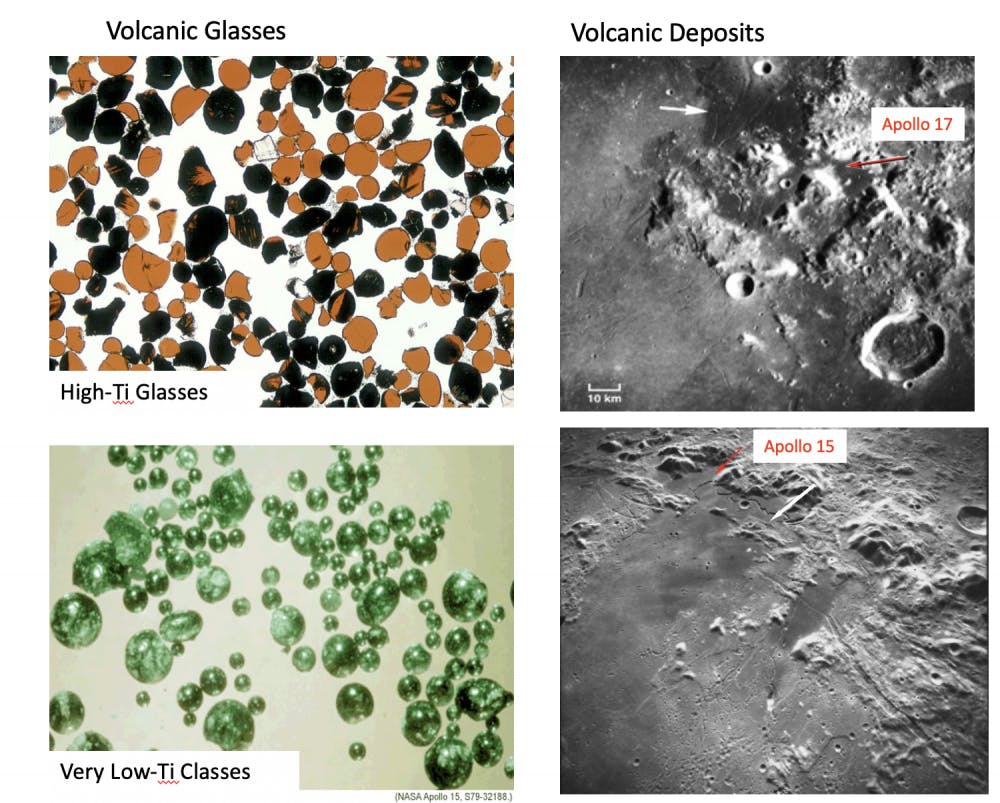
Professor of Earth, Environmental and Planetary Sciences Alberto Saal and his colleague, late Carnegie scientist Erik Hauri analyzed sulfur isotopes in samples of volcanic glass collected during NASA’s Apollo 15 and 17 missions to understand the Moon’s formation and early history in a study published Feb. 24.
What is known about the formation of the moon, Saal said, is that an impact event pulverized both a part of the Earth and another planet. This collision formed a disc of debris around the Earth which began to coalesce to form the Moon.
Due to this impact, the Moon serves as a “comparative planet” because it can reveal information about the early history of Earth, Professor of Geological Sciences Jim Head said.
Currently, only a very small portion of Earth’s history is known. “Imagine the radius of the earth as 6,000 km. We have only seen, directly, samples that come from only about the first 100 km, maybe 200 km,” Saal said.
Many unanswered questions revolve around what the composition of the rest of Earth’s core is like. Unlike Earth, the Moon has essentially been frozen due to low gravity and its large surface area to volume ratio so the moon’s composition has remained similar to that of early Earth, Head said. Samples from the Moon will not only provide evidence of what happened during its formation, but they can also serve as a way to learn about the early history of the earth.
For years, the Moon was believed to not have many volatile elements. “Volatile elements are elements that like to be in a gas phase,” Saal said. These include hydrogen, carbon, chlorine, fluorine and sulfur. In the paper, Saal refutes this belief. He proposes that processes have changed the lava compositions of the Moon, resulting in the reduced amount of gas found in surface samples.
Saal concluded that the responsible process is degassing, which occurred when reservoirs in the Moon erupted out of its surface as lava, and gases escaped from the lava due to the absence of gas in the atmosphere. After accounting for those processes, the isotope ratios in volcanic glass samples were found to be very similar to those on Earth, Saal said.
Along with analyzing volcanic glass, which is cooled lava, Saal’s lab also analyzed the interiors of olivine-hosted melt-inclusions, small samples of magma trapped in an olivine crystal that have not been exposed to the Moon’s atmosphere. Using the volcanic glass samples and melt-inclusions, Saal showed that after accounting for degassing, the sulfur isotopes from these samples are much more similar to the sulfur isotope ratios of Earth than previously thought.
A great strength of Saal’s paper is his use of secondary ion mass spectrometry, called NanoSIMS technology, in collecting data, said Kei Shimizu, a former PhD student in Saal’s lab and a current postdoctoral researcher at University of Wisconsin-Madison. NanoSIMS technology allows for very precise characterization of isotope ratios.
Saal said NanoSIMS technology allows the lab to measure very small amounts of material while avoiding more contamination and achieving a greater degree of specificity. With this method, he found that the concentration of sulfur decreases from the core to the rim of the volcanic glass sample, indicating that elements are being lost from the glass. This finding debunks the theory that the gas found in samples from previous studies was due to contamination by Earth’s atmosphere, Saal said.
Though Saal’s paper did not completely answer the question of whether the Moon and Earth originated from the same material, it showed that processes like degassing very likely have affected the current ratios of the Moon’s sulfur isotopes. In addition, the processes Saal’s paper proposed to explain the chemical composition of samples are consistent with many prior observations from previous studies, Saal said.
This paper was of special significance to Saal because he co-authored the study with the late Erik Hauri, whom he had worked with since 2007. Hauri died in 2018. He was an “extremely good analyst,” Saal said, not only because of his genius but also because he was so open to constructive debate. The most difficult part of this study was restarting the analysis without Hauri, he added.
In the future, Saal’s lab plans to test his theories from this paper by doing the same experiment with other isotopes. Head and Shimizu hope that Saal’s lab will investigate more lunar samples, such as the relatively young samples from the recent Chinese Chang’e-5 lunar mission.
Learning about Earth’s early history is not just a dive into the past, Head said. Addressing fundamental questions, such as the origin of life, the evolution of life and how our planet works, will give us clues about our future and our changing planet, he added.
ADVERTISEMENT
More




