A team of University researchers has proposed a new potential target for cancer treatments.
The researchers identified the target — a structural protein known as vimentin — by observing a connection between a particular subset of cancer cells’ aggressiveness and their reliance on this protein for travel throughout the body. The researchers’ findings, published last month, build on a project that began about two years ago.
The subset of cancer cells that the group studied, called Polyploidal Giant Cancer Cells, is one that has often been ignored because of variation from most other cancer cells. But there are “a lot of really important qualities that make them important to study and understand on a level of developing new treatment strategies to target invasive cancer cells,” said Principal Investigator Michelle Dawson, assistant professor of biomedical sciences.
PGCCs, named for the enlarged size of their nuclei, undergo a unique replication process, Dawson said. Unlike other cancer cells that replicate through mitosis with the final step of cytokinesis — the process during which a cell with twice its typical DNA content separates into two daughter cells — these PGCCs divide by a different process called amitotic budding. The cells continue to synthesize DNA for a longer period of time before dividing, resulting in their enlarged structures.
Targeting these cells becomes challenging in a clinical setting, where many of the drugs used to treat cancer target mitosis.
PGCCs are also able to survive despite DNA damage induced through typical cancer treatments like platinum drugs or radiation, Dawson said. Researchers hypothesize that amitotic budding accounts for their survival because the damaged parts of the cell will be able to bud off.
According to a separate 2014 study published in Oncogene by a team of researchers from the University of Texas MD Anderson Cancer Center and from Anhui Medical University and Tianjin Medical University and Tianjin Cancer Institute in China, PGCCs “play a fundamental role in regulating tumor heterogeneity, stemness and chemoresistance in human cancer,” making these cells more aggressive and harder to treat than other cancer cells.
The Brown team’s previous paper had shown that PGCCs are highly chemoresistant and are ubiquitous across cancer cell lines, The Herald previously reported. In the study published this year, the researchers sought to investigate the underlying mechanism of the cells’ aggressiveness, thereby finding the connection to vimentin, Dawson said.
Vimentin is a protein found in many cell types and is an intermediate filament — these filaments are molecules inside a cell that join together in a network to reinforce the cell’s structure. This network is known as the cytoskeleton. But more of the protein is produced in epithelial cancer cells — epithelial cells are those surrounding body organs — that become more migratory and invasive. Vimentin is therefore associated with cancer progression, Dawson explained.
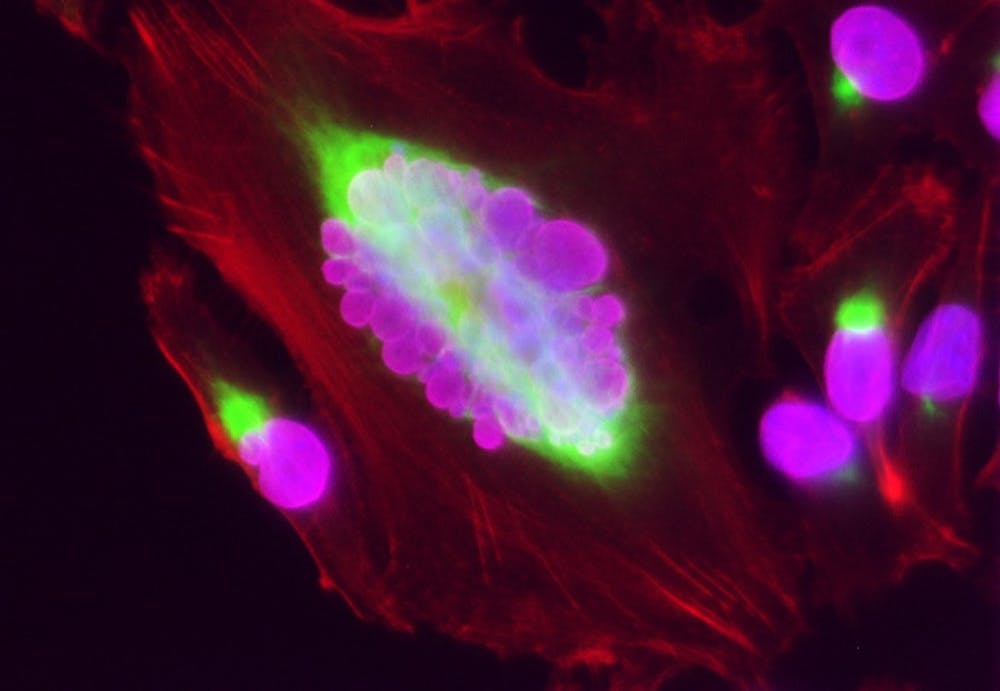
The research team used a cellular staining technique known as immunofluorescence — in which components of interest in the cell produce a colorful glow under a microscope — to confirm their prior findings showing higher levels of vimentin in PGCCs than in other cells. This observation led the researchers to believe that vimentin helps maintain the enlarged structure of PGCCs.
The team’s observations also suggested that vimentin played a role in PGCCs’ persistent migration — the cancer cells’ movement in a straight line — which is unique to these types of cancer cells, Dawson said.
To test their hypothesis, the group relied on a technique called the scratch wound assay. The researchers grew cancer cells over a surface, removed a region of the surface and allowed the cells to fill that region to measure the cells’ potential to migrate, said lead author of the study Botai Xuan GS, a PhD candidate in molecular pharmacology and physiology.
As the cells were migrating into the removed region, the researchers used automated technology to measure each individual cell’s movement, Xuan said. The group tracked the motion of the cells and identified which ones were PGCCs as opposed to non-PGCC cancer cells.
The group found that PGCCs were most likely to close the gap and that vimentin was primarily located at the leading edge of the cell, which is important to understanding the movement of the cells, Dawson said.
When the team eliminated vimentin from the PGCCs, the filament structures collapsed, disrupting PGCC migration so that the cells were no longer able to close the gap. The team determined that PGCCs “rely on vimentin to maintain their cell volume and migratory persistence and potential,” Xuan said.
The researchers’ study ultimately showed that PGCCs have more migratory potential due to support from the vimentin network. The findings are significant because metastasis — the ability of cancer cells to grow beyond their original location — “is the number one cause of cancer death,” making the targeting of migratory behaviors necessary to improving patient outcomes, Xuan said.
The other researchers involved with the project were Postdoctoral Research Associate Deepraj Ghosh, Rachelle Shao ’22 and Joy Jiang ’19.
“I am extremely grateful to have been able to perform cutting-edge research under (Dawson’s) mentorship and that of Botai Xuan,” Jiang wrote in an email to The Herald.
The team is continuing with follow-up studies, Xuan said. The group hopes to look at PGCCs in a three-dimensional environment to visualize how they may interact with their surroundings to promote increased invasion and malignant transformation.
ADVERTISEMENT




