When life-saving drugs wind through catheters and into the drifting current of the bloodstream, sometimes unwanted and even deadly bacteria tag along, prepared to invade and infect vulnerable immune systems.
But now, a life-saving drug can be combined with a form of plastic to defend the border between human blood and the IV catheter, fighting infections before they occur. The new antibacterial coating, designed in a study led by researchers at the University and the Warren Alpert Medical School, can slowly release the anti-microbial drug auranofin to kill microbes and bacteria on the catheter’s edge, said Assistant Professor of Medicine (Research) Beth Fuchs. The research has not yet been conducted on human subjects.
Fuchs and Professor of Infectious Diseases and Chief of the Infectious Disease Division of Rhode Island Hospital Eleftherios Mylonakis discovered that auranofin can be used to kill bacteria and reduce the rate of bacterial infection in worms. Then, Anita Shukla, assistant professor of engineering and molecular pharmacology, physiology and biotechnology, led the process of designing the catheter coating to deliver the drug.
A common and often dangerous culprit of catheter-related bloodstream infections is MRSA, methicillin-resistant Staphylococcus aureus bacteria. These bacteria resist common antibiotics and thus are a challenge to eliminate in the human body, Fuchs said.
MRSA is prevalent in hospitals where catheters are typically inserted into patients. While the bacteria are common, infections often occur because catheters provide the bacteria with a gateway into the body, and patients who may already have compromised immune systems can have a harder time fighting off the infections, Fuchs said. U.S. data points to 250,000 catheter-related bloodstream infections per year, she said.
Currently, treatment for MRSA and other catheter-based bloodstream infections requires removing the patient’s catheter and then treating the infection with antibiotics administered intravenously for an extended period of time, Mylonakis wrote in an email to The Herald.
This new research has shown that a unique combination of auronafin and polymer could be the solution to this problem.
Unlike past attempts to make antibacterial catheters, this invention allows the drug to be released from a period as short as one week to as long as 26 days, Fuchs said. Delivering auranofin slowly is especially important given the fact that some catheters may need to be kept in place for a substantial period of time, which increases the likelihood that an infection can occur after insertion.
Because blood is water-based and auranofin is hydrophobic, or water-fearing, the researchers knew the drug would release gradually into the body at sites prone to infection, Shukla said. Because the drug is delivered slowly over time, the amount of medication a patient needs to treat an infection may go down. In turn, this may reduce the growth of drug-resistant bacteria because these bacteria develop their resistance over time and to higher doses of drug exposure, Fuchs added.
The drug may also be able to prevent the formation of biofilm, which is the collection of bacteria that can stick to plastic catheters and collectively withstand antibiotics, Fuchs said. Currently, it is quite difficult to maintain clean catheters.
Auranofin’s antibiofilm properties are “really one of the most desirable features” of the catheters, and without that particular drug, the coating is not as effective, Shukla said. When the team tried to use a different drug — vancomycin — the catheter did not exhibit the same properties. The collective benefits of this particular coating mean that this invention is “perhaps more successful than some of the previous attempts” to make antibacterial catheters, Shukla said.
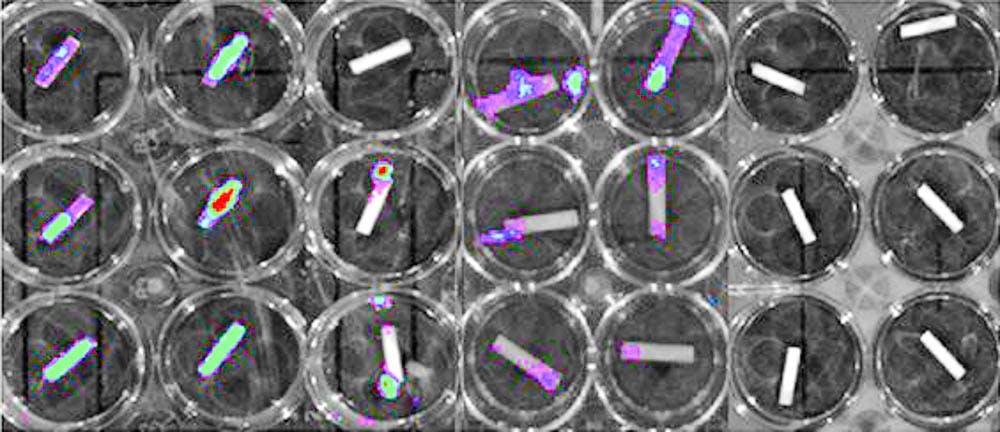
To test the efficacy of the catheters, the researchers soaked them in a solution containing MRSA and used bioluminescent MRSA to determine how well the coating killed the bacteria and prevented biofilm formation, Shukla said.
“It’s been really great working with the people over in the hospital” and “getting to learn more about the different perspectives of both type of researchers … It brings together a lot of different expertise,” Shukla said.
The researchers now hope to take the in vitro experiment from the Petri dish and apply it to mouse models, Fuchs said. Researchers also aim to test other drugs in an effort to discover those that could target bacterial infections that do not respond well to auranofin.
The researchers have not yet “seen any adverse effects;” the drug is FDA-approved. The team was “very pleased with the results.”
Beyond catheters themselves, the team’s combination of drug and polymer could also prove effective on materials like orthopedic implants, Shukla added.